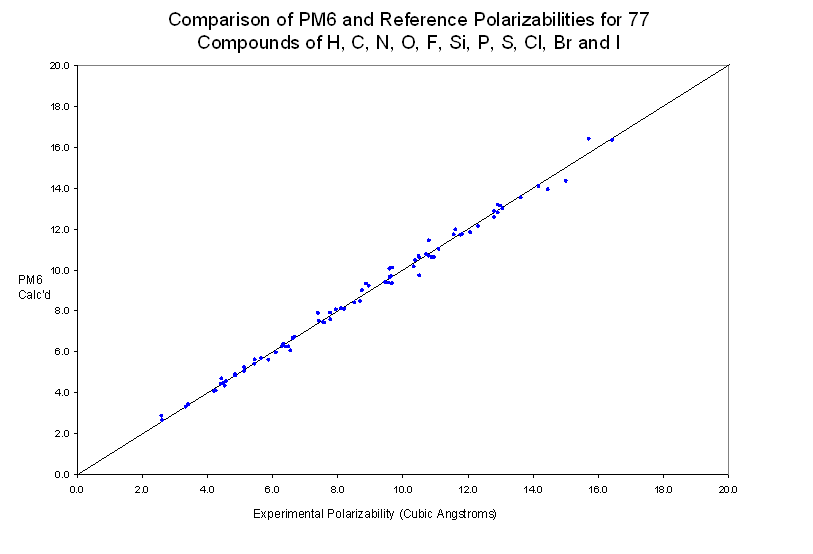
By use of a keywords "POLAR" and "STATIC" the polarizability of a molecule can be calculated. For a set of 77 neutral molecules, the average unsigned error was 2.1%.
"STATIC" uses a set of applied electric fields to evaluate the effect on the calculated heat of formation and on the dipole. From the resulting three by three tensor, the polarizability along three orthogonal axes can be evaluated. A measure of the precision of the calculation can be obtained by comparing the polarizability calculated by the two methods.
"POLAR" evaluates the frequency dependent response of a molecule. The response can be expressed as the series expansion: Charge, dipole, polarizability (alpha), beta and gamma (the first and second hyperpolarizabilities)
The calculated polarizability is modified to include atomic correction terms. Given a calculated polarizability of "R", the corrected polarizability is give as:
Polarizability = 0.262114*H+0.485071*C+0.154301*O+0.204743*N+1.45331*S+0.199611*F+1.23621*Cl+2.14242*Br+3.82316*I+2.31461*P+1.88611*Si+0.791396*R+0.373638
where "H", "C", etc. are the number of hydrogen, carbon, etc. atoms in the molecule.
For the 77 compounds used, the value of R2 is 0.9942 and the cross-validated R2 is 0.9901.

Individual Species used in Preparing this Graph
(in order of increasing polarizability)
|
Chemical |
Exp. alpha | PM6 | P-C | Abs(PM6-Exp) |
|
Fluoromethane |
2.590 | 2.878 | 0.288 | 0.288 |
|
Methane |
2.600 | 2.672 | 0.072 | 0.072 |
|
Acetylene |
3.330 | 3.322 | -0.008 | 0.008 |
|
Formic acid |
3.400 | 3.438 | 0.038 | 0.038 |
|
Formamide |
4.200 | 4.076 | -0.124 | 0.124 |
|
Ethylene |
4.250 | 4.119 | -0.131 | 0.131 |
|
Acetonitrile |
4.400 | 4.440 | 0.040 | 0.040 |
|
Fluoroethane |
4.430 | 4.698 | 0.268 | 0.268 |
|
Ethane |
4.470 | 4.481 | 0.011 | 0.011 |
|
Methyl chloride |
4.530 | 4.339 | -0.191 | 0.191 |
|
Acetaldehyde |
4.570 | 4.579 | 0.009 | 0.009 |
|
Sulfur trioxide |
4.840 | 4.841 | 0.001 | 0.001 |
|
Phosphine |
4.840 | 4.909 | 0.069 | 0.069 |
|
Ethanol |
5.110 | 5.063 | -0.047 | 0.047 |
|
Acetic acid |
5.130 | 5.247 | 0.117 | 0.117 |
|
Silane |
5.440 | 5.419 | -0.021 | 0.021 |
|
SiF4 |
5.450 | 5.624 | 0.174 | 0.174 |
|
Dimethylamine |
5.650 | 5.697 | 0.047 | 0.047 |
|
Ethylamine |
5.870 | 5.616 | -0.254 | 0.254 |
|
Phosphorus pentafluoride |
6.100 | 5.981 | -0.119 | 0.119 |
|
Propane |
6.290 | 6.276 | -0.014 | 0.014 |
|
Acetone |
6.330 | 6.393 | 0.063 | 0.063 |
|
Chloroethane |
6.410 | 6.260 | -0.150 | 0.150 |
|
Dichloromethane |
6.480 | 6.264 | -0.216 | 0.216 |
|
Sulfur hexafluoride |
6.540 | 6.077 | -0.463 | 0.463 |
|
Fluorodichloromethane |
6.610 | 6.667 | 0.057 | 0.057 |
|
Acetyl chloride |
6.670 | 6.728 | 0.058 | 0.058 |
|
Bromochlorofluoromethane |
7.390 | 7.896 | 0.506 | 0.506 |
|
Thioethanol |
7.410 | 7.515 | 0.105 | 0.105 |
|
Bromoethane |
7.540 | 7.446 | -0.094 | 0.094 |
|
Bromochloromethane |
7.580 | 7.428 | -0.152 | 0.152 |
|
Fluoroiodomethane |
7.760 | 7.925 | 0.165 | 0.165 |
|
Trimethylamine |
7.770 | 7.591 | -0.179 | 0.179 |
|
Acetyl bromide |
7.940 | 8.077 | 0.137 | 0.137 |
|
alpha-Bromoacetic acid |
8.100 | 8.147 | 0.047 | 0.047 |
|
n-Butane |
8.200 | 8.083 | -0.117 | 0.117 |
|
Chloroform |
8.510 | 8.409 | -0.101 | 0.101 |
|
Dibromomethane |
8.690 | 8.473 | -0.217 | 0.217 |
|
Dibromofluoromethane |
8.740 | 9.011 | 0.271 | 0.271 |
|
Acetic anhydride |
8.870 | 9.324 | 0.454 | 0.454 |
|
1,1-Dichloro-1,2,2,2-tetrafluoroethane |
8.950 | 9.231 | 0.281 | 0.281 |
|
1-Bromo-2-chloroethane |
9.460 | 9.416 | -0.044 | 0.044 |
|
Pyridine |
9.540 | 9.380 | -0.160 | 0.160 |
|
Bromodichloromethane |
9.590 | 9.644 | 0.054 | 0.054 |
|
Diethyl ketone |
9.590 | 10.060 | 0.470 | 0.470 |
|
Chloroiodomethane |
9.640 | 9.696 | 0.056 | 0.056 |
|
Diethylamine |
9.660 | 9.352 | -0.308 | 0.308 |
|
Thiophene |
9.670 | 10.118 | 0.448 | 0.448 |
|
Benzene |
10.330 | 10.167 | -0.163 | 0.163 |
|
Acetyl iodide |
10.370 | 10.486 | 0.116 | 0.116 |
|
Carbon tetrachloride |
10.480 | 10.668 | 0.188 | 0.188 |
|
2,2,2-Trichloroacetaldehyde |
10.500 | 10.619 | 0.119 | 0.119 |
|
SO2Cl2 |
10.500 | 9.726 | -0.774 | 0.774 |
|
Dibromochloromethane |
10.710 | 10.760 | 0.050 | 0.050 |
|
Bromoiodomethane |
10.790 | 10.683 | -0.107 | 0.107 |
|
Diethyl thioether |
10.800 | 11.433 | 0.633 | 0.633 |
|
1,1-Dibromoethane |
10.890 | 10.620 | -0.270 | 0.270 |
|
Trichloroacetic acid |
10.960 | 10.619 | -0.341 | 0.341 |
|
Disilane |
11.100 | 11.024 | -0.076 | 0.076 |
|
Cyclohexanol (eq) |
11.560 | 11.727 | 0.167 | 0.167 |
|
Trichlorobromomethane |
11.610 | 11.987 | 0.377 | 0.377 |
|
n-Hexane |
11.760 | 11.717 | -0.043 | 0.043 |
|
Bromoform |
11.820 | 11.760 | -0.060 | 0.060 |
|
Aniline |
12.070 | 11.835 | -0.235 | 0.235 |
|
Toluene |
12.310 | 12.133 | -0.177 | 0.177 |
|
Phosphorus trichloride |
12.800 | 12.850 | 0.050 | 0.050 |
|
Benzaldehyde |
12.800 | 12.577 | -0.223 | 0.223 |
|
Diiodomethane |
12.910 | 12.794 | -0.116 | 0.116 |
|
Phenylhydrazine |
12.910 | 13.185 | 0.275 | 0.275 |
|
Anisole |
12.980 | 13.130 | 0.150 | 0.150 |
|
m-Cresol |
13.050 | 12.999 | -0.051 | 0.051 |
|
n-Heptane |
13.610 | 13.540 | -0.070 | 0.070 |
|
2,2,2-Tribromoacetaldehyde |
14.170 | 14.099 | -0.071 | 0.071 |
|
Styrene |
14.450 | 13.936 | -0.514 | 0.514 |
|
Acetophenone |
15.000 | 14.346 | -0.654 | 0.654 |
|
Quinoline |
15.700 | 16.406 | 0.706 | 0.706 |
|
Isoquinoline |
16.430 | 16.348 |
-0.082 |
0.082 |
|
Totals |
672.500 | 14.274 |