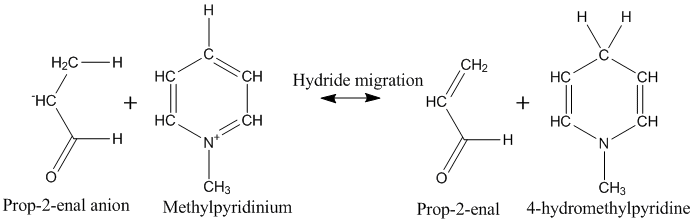

|
In enzyme-catalyzed dehydrogenation, the hydride transfer reaction occurs after a proton has been abstracted from the substrate, resulting in the formation of an anionic species. In this simulation the substrate is represented by prop-2-enal anion. Hydride transfer typically involved the oxidized form of nicotinamide adenine dinucleotide, NAD+, in the simulation this is represented by a methylpyridinium cation. Addition of a hydride to NAD+ converts it into the reduced form, NADH, represented here by 4-hydromethylpyridine
The components are, on the left-hand side, prop-2-enal anion and methylpyridinium cation, and, on the right-hand side neutral prop-2enal and 4-hydromethylpyridine. Because of the charges present, the simulation is done using the COSMO solvation model.
Before starting any work on the reaction, the geometries of both the reactants and the products were fully optimized. In the reactants, this positioned the -CH3 group of the prop-2-enal anion near to C4 of methylpyridinium, and the rest of the prop-2-enal anion was oriented across the face of the pyridinium, maximizing the electrostatic stabilization resulting from the very large charge separation. That electrostatic stabilization is not present in the products, so the two species move apart, but only slightly.
At the transition state, the migrating hydrogen atom is positioned approximately mid-way between the carbon atom it came from and the carbon atom it is moving towards.
From the title "hydride migration" the assumption might be made that the migrating hydrogen atom would have a large negative atomic charge, however, both semiempirical, here PM7, and B3LYP 6-311G predict a small positive charge on the migrating atom. The obvious question then arises of how does the negative charge move from one species to the other? An examination of the Intrinsic Reaction Coordinate (IRC) shows that the net charge on the anion remains almost unchanged until the top of the barrier is reached, and that in that in the region just before the transition state, at the transition state, and just after the transition state, there is a rapid movement of charge from one species to the other. That is, the movement of charge occurs when the migrating hydrogen atom forms a bridging bond between the two species. At that point, the PM7 partial charges are, on the prop-2-enal: -0.52 (-0.52), on the hydrogen atom +0.13 (+0.22), and on the methylpyridine: +0.39 (+0.30). The partial charges in parentheses are those predicted by B3LYP 6-311G.
 Reaction
barrier
Reaction
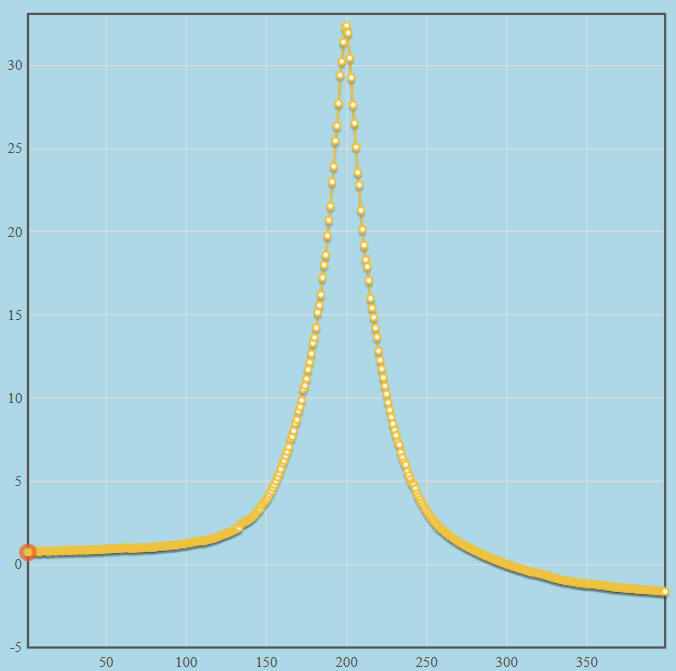
barrierThe IRC for the reaction is shown in the nearby chart. Each point calculated in the IRC is shown as a yellow dot, and a smoothed line through these points is also shown in yellow. Only 200 points were generated on each side of the transition state, so the starting point on the left-hand side is +0.72 kcal·mol-1, versus the optimized ΔHf of the reactants of -0.97 kcal·mol-1. The difference was due to the truncation of the IRC. Similarly, on the right-hand side, the chart ends at -1.62 kcal·mol-1 versus the optimized ΔHf of the products of -2.74 kcal·mol-1.
The "distance" from the start of the left-hand side to the transition state is 4.39 Ångstroms, and from the transition state to the right-hand side is 5.02 Ångstroms, for a total chart-width of 9.41 Ångstroms. Within this space, the barrier itself has a width of only about 25% or 2.35 Ångstroms. Given that the height of the barrier is about 30 kcal·mol-1, this is a very steep barrier. This is reflected in the large value of the imaginary frequency for the transition state normal mode, of i2472 cm-1. The appearance of the barrier is typical of both a proton and a hydrogen atom transfer.